Search the Community
Showing results for tags 'Pfizer'.
-
Pfizer says its experimental pill reduces risk of hospitalization, death from Covid-19 By Maggie Fox, and Amanda Sealy, CNN Updated 1045 GMT (1845 HKT) November 5, 2021 (CNN)Drugmaker Pfizer said Friday its experimental pill designed to fight coronavirus reduced the risk of hospitalization and death for high-risk patients taking part in a trial of the drug. The company hopes it can eventually offer the pill, given in combination with an older antiviral drug called ritonavir, to people to take at home before they get sick enough to go to the hospital. A so-called interim analysis -- done before the trial was scheduled to end -- showed an 89% reduction in the risk of hospitalization or death from Covid-19 if patients got it soon enough, the company said. Pfizer released the results in a news release and did not provide scientists to discuss the data ahead of release. The data has not been peer reviewed or published. The company says it will share more specifics in a peer-reviewed paper and with its submission to the US Food and Drug Administration. The company has been testing the drug in adults with Covid-19 who are considered at high risk of progressing to severe illness. The volunteers have been randomly given either the pill combination or a placebo within three days or five days of their symptoms starting. The pill, still known by its experimental name PF-07321332, is what's known as a protease inhibitor. It's designed to stop the virus from multiplying. Giving it along with ritonavir slows its breakdown in the body, the company said. Pfizer said 0.8% of patients who got the drug combination within three days were hospitalized within four weeks -- three out of 389 patients -- compared to 7% of patients who got placebos, or 27 out of 385. And seven of those who got placebos died, Pfizer said. No one who got the treatment died within a month. "Similar reductions in COVID-19-related hospitalization or death were observed in patients treated within five days of symptom onset; 1% of patients who received PF-07321332 (with) ritonavir were hospitalized through Day 28 following randomization (6/607 hospitalized, with no deaths), compared to 6.7% of patients who received a placebo," the company said. It said 19% of patients given the treatment suffered adverse events, compared to 21% who got placebo, but declined to disclose what those adverse events were. "These data suggest that our oral antiviral candidate, if approved by regulatory authorities, has the potential to save patients' lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations," Albert Bourla, chairman CEO of Pfizer, said in a statement. Currently, remdesivir, sold under the brand name Veklury, is the only antiviral approved by FDA for treatment of Covid-19. It's given by intravenous infusion, so it's not as simple to administer as a pill. People can also be treated with monoclonal antibodies, which are injected or infused therapies that kickstart the immune system to help fight off infection. They are not as easy to take as a pill and must be administered by a trained professional. Merck is seeking FDA emergency use authorization for molnupiravir, an antiviral capsule people could take at home. It's been shown to reduce the risk of hospitalization or death by about 50%. On Thursday, UK drug regulators authorized molnupiravir under the brand name Lagevrio.
- 34 replies
-
- 2
-

-
- pfizer
- experimental
-
(and 3 more)
Tagged with:
-
https://www.ndtv.com/world-news/norway-warns-of-vaccination-risks-for-sick-elderly-patients-after-23-die-2353257 Norway Warns of Vaccination Risks For Sick Elderly Patients After 23 Die Norwegian officials said 23 people had died in the country a short time after receiving their first dose of the vaccine. Of those deaths, 13 have been autopsied, with the results suggesting that common side effects may have contributed to severe reactions in frail, elderly people, according to the Norwegian Medicines Agency. Pfizer and BioNTech are working with the Norwegian regulator to investigate the deaths in Norway, Pfizer said in an e-mailed statement. The agency found that "the number of incidents so far is not alarming, and in line with expectations," Pfizer said. Allergic reactions have been uncommon so far. In the U.S., authorities reported 21 cases of severe allergic reactions from Dec. 14-23 after administration of about 1.9 million initial doses of the vaccine developed by Pfizer Inc. and BioNTech SE. That's an incidence of 11.1 cases per million doses, according to the Centers for Disease Control and Prevention. Though both Covid-19 vaccines approved so far in Europe were tested in tens of thousands of people -- including volunteers in their late 80s and 90s -- the average trial participant was in his or her early 50s. The first people to be immunized in many places have been older than that as countries rush to inoculate nursing-home residents at high risk from the virus.
-
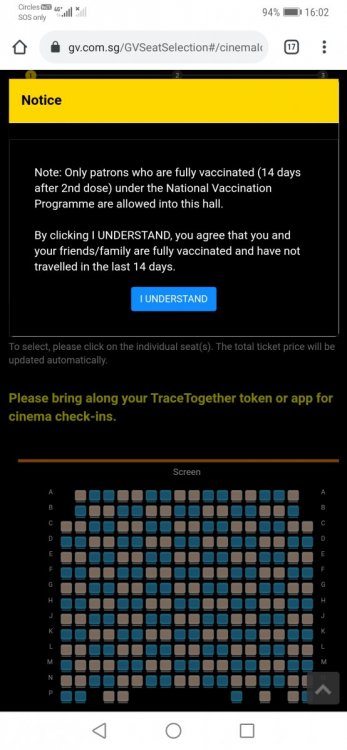
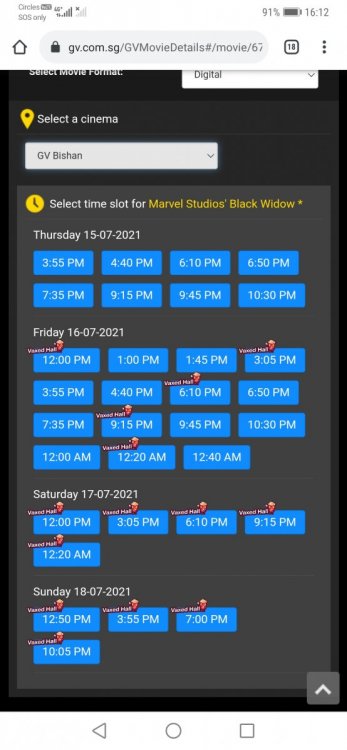
Second vaccine arrive in Singapore yesterday. I would like to start this thread to consolidate feedback on Moderna vaccine for everyone's benefit. As I understand we will not be able to choose the brand of vaccine,my question is although we cannot choose,but we will be able to know which vaccine we are being administered with right,since there is a leaflet given to you at the vaccination centre indicating side effects etc etc etc... Side effects for Moderna reportedly stronger than Pfizer but comparatively most of the news with serious side effects or "deaths" reported mostly like belong to Pfizer. Regardless,I'm all for vaccination if it saves lives.... Good job 🇸🇬 Singapore received its first shipment of Moderna's COVID-19 vaccine on Wednesday (Feb 17), two weeks after authorities approved it for use here. The vaccines were carried on board Singapore Airlines (SIA) flight SQ7137, a scheduled freighter service from Brussels, Belgium, and arrived in Singapore at about 1.40pm. "The vaccines were prioritised for loading into the aircraft in Brussels and was given precedence during unloading in Singapore. They were then transported to SATS' cold-chain facility, Coolport, for subsequent storage and ground transportation," SIA said on Wednesday. It was announced on Feb 3 that the Health Sciences Authority has granted interim authorisation for Moderna's COVID-19 vaccine to be used in Singapore for people aged 18 years and above. This is the second vaccine approved for use in Singapore.
-
If You Got Pfizer, You May Not Have Antibodies Against Delta After This Long (yahoo.com) https://www.yahoo.com/lifestyle/got-pfizer-may-not-antibodies-103253108.html Durability of immune responses to the BNT162b2 mRNA vaccine | bioRxiv https://www.biorxiv.org/content/10.1101/2021.09.30.462488v1 That means Pfizer vaccines T cell response to Covid19 also wane to below detectable level. Pfizer/mRNA is like "useless". Look at all the daily new cases here almost all had already been vaccinated with 2 doses of Pfizer. And Pfizer doesn't prevent you from death if kena Covid-19 as daily updates shown
-
Such episode gives Chinese a bad name. :/ Pfizer employee ‘stole vaccine secrets’. https://www.rt.com/usa/541251-pfizer-employee-vaccine-secrets/ Pfizer has accused a departing employee of making off with a massive trove of confidential documents, including trade secrets related to its Covid-19 vaccine and other drugs, and then attempting an elaborate cover-up. The Big Pharma firm launched a suit against the staffer, identified as Chun Xiao Li, earlier this week, alleging that she “uploaded over 12,000 files – including scores of confidential Pfizer documents – from her Pfizer-issued laptop to a personal Google Drive account” in late October, and then transferred those files to her own private device. “Due to the sheer number of documents Ms. Li misappropriated, Pfizer has yet to understand the full scope of trade-secret and confidential information in her possession,” the company said, adding that while Li now possesses “thousands of documents potentially related to numerous Pfizer vaccines,” the lawsuit is focused on its Covid-19 immunization and monoclonal antibody therapies. "Given her role and responsibilities as Associate Director of Statistics, Ms. Li had access to highly confidential, proprietary, and trade-secret information related to numerous vaccines and medications, including the Covid-19 vaccine..." Pfizer also alleges that Li worked with up to five unidentified co-conspirators, and even went as far as to provide a “decoy laptop” to mislead the company’s security team, leaving them to believe it was the device used to exfiltrate the files. However, Pfizer said “forensic analyses” confirmed it was not the same laptop, and that Li – or one of her accomplices – “likely remains in possession of the actual computer that contains those 12,000 files.” After discovering the document heist, the company said it conducted a review of Li’s emails, file access and web activity on her company-issued laptop, finding that she had been in discussions with a rival pharma firm, Xencor, about a job opportunity. Earlier this month, as Pfizer continued its probe, Li informed superiors that she would be leaving the company, but did not disclose any reason for doing so, including any job offer at Xencor – despite the fact she had already been hired there, and was set to begin work on November 29. Given that Li is still thought to possess a large trove of the company’s trade secrets, Pfizer argued that it would be “unjust to permit Ms. Li and anybody with whom she may be working in concert to trade on Pfizer’s successes and experience, whether at Xencor or elsewhere, by leveraging the numerous confidential Pfizer documents she took without permission and refuses to return,” and is therefore seeking a restraining order to bar her from using the material for “her own purposes.” Despite those allegations, Li has insisted that she’s provided all information requested by Pfizer and cooperated with its investigation, according to the court filing, which also noted that Li has declined further meetings with the company due to unspecified “health issues.”
-
I think it's a good read to understand more of what mRNA is about, and maybe infer its safety Could mRNA make us superhuman? Until recently most people had never even heard of mRNA vaccines. Now scientists believe they may be the key to solving a wealth of health problems. Barely a year ago, Anna Blakney was working in a relatively inconspicuous, niche field of science in a lab in London. Few people outside of her scientific circles had heard of mRNA vaccines. Because none yet existed. Attendees at an annual conference talk she gave in 2019 could be counted in the tens, not hundreds. Today, she's in hot demand: an assistant professor at the University of British Columbia, Canada, and a science communicator with 253,000 followers and 3.7 million likes on TikTok. She was, she admits, in the right place at the right time to ride a once-in-a-generation wave of scientific progress. She even gave this new era a name: "the RNAissance". Due to the Covid-19 pandemic, many people have now heard of – and have received – an mRNA vaccine, from the likes of Pfizer-BioNTech and Moderna. But even when Blakney started her PhD at Imperial College London in 2016, "a lot of people were sceptical as to whether it could ever work". Now, "the whole field of mRNA is just exploding. It's a game changer in medicine," she says. It's such a game changer that it raises some very big, exciting questions: could mRNA vaccines provide a cure for cancers, HIV, tropical diseases, and even give us superhuman immunity? Messenger ribonucleic acid, or mRNA for short, is a single-stranded molecule that carries genetic code from DNA to a cell's protein-making machinery. Without mRNA, your genetic code wouldn't be used, proteins wouldn't be made, and your body wouldn't work. If DNA is the bank card, then mRNA is the card reader. Once a virus is inside our cells, it releases its own RNA, tricking our hijacked cells into spewing out copies of the virus – in the form of viral proteins – that compromise our immune system. Traditional vaccines work by injecting inactivated virus proteins called antigens, which stimulate the body's immune system to recognise the virus when it reappears. The genius of mRNA vaccines is there's no need to inject the antigen itself. Instead, these vaccines use the genetic sequence or "code" of the antigen translated into mRNA. It's a ghost of the real thing, fooling the body into creating very real antibodies. The artificial mRNA itself then disappears, degraded by the body's natural defences including enzymes that break it down, leaving us with only the antibodies. It is, therefore, safer to produce, more quickly and cheaply, compared with traditional vaccines. You no longer need huge bio-secure labs growing deadly viruses inside millions of chicken eggs. Instead, just one lab can sequence the proteins of the antigen and email it around the world. With that information a lab could make "a million doses of mRNA in a single 100ml test tube," says Blakney. We've now seen that process play out in real time. On 10 January 2020, Zhang Yongzhen, a professor of zoonoses at the Chinese Centre for Disease Control and Prevention in Beijing sequenced the genome for Covid-19 and published the next day. Covid-19 was declared a pandemic by the World Health Organization (WHO) on 11 March. On 16 March, using Zhang's sequence, the first mRNA vaccine began its phase one clinical trial. The US Food and Drug Administration approved the Pfizer-BioNTech Covid-19 vaccine on 11 December, 2020, making history as not only the first ever mRNA vaccine approved for humans but also as the first to have a 95% efficacy rate in clinical trials. Approval of the Moderna mRNA vaccine followed close behind on 18 December. The previous title holder for "fastest ever vaccine", the mumps vaccine, took four years. The Moderna and Pfizer–BioNTech vaccines took just 11 months. The theory behind the mRNA vaccine was pioneered by University of Pennsylvania scientists Katalin Karikó and Drew Weissman, who both recently received the 2021 Lasker Award, America's top biomedical research prize. Even in 2019, however, mainstream mRNA vaccines were believed to be at least five years away. The pandemic fast-forwarded this field of medicine by half a decade. Kathryn Whitehead, an associate professor of chemical engineering and biomedical engineering at Carnegie Mellon University, and a key collaborator of Weissman and Karikó admits, "there weren't many people in the mRNA therapeutics world who would have imagined 95% initial efficacy rates in this emergency scenario". But now, the possibilities are seemingly endless. Or, as Blakney puts it: "Now it's like, OK, so it's worked for a viral glycoprotein, what other vaccines can we make with it? And what can we do beyond that?" At the University of Rochester, Dragony Fu, associate professor, department of biology, received expedited funding for his laboratory from the National Science Foundation to research RNA proteins. If we are currently witnessing mRNA vaccine 1.0 for Covid-19, then 2.0 will address two further categories of disease, says Fu: "one is pathogens, like Sars, but you can apply this technology to other foreign invaders such as HIV. Already before Covid, companies were in development making mRNA vaccines against HIV." He also cites Zika, herpes and malarial parasites in the pathogens camp. "The other category is autoimmune diseases," he says. "That is intriguing because it's verging beyond the very strict definition of a vaccine." Fu says the future could involve mRNA "treatments", for example to reduce inflammation. "In theory, that opens up so many possibilities," he says. Yizhou Dong, associate professor of pharmaceutics and pharmacology Ohio State University, specialises in little balls of fat, or lipids, needed to house the mRNA and safely deliver it to the cells without being immediately destroyed by our body. Lipids have been described as the "unsung hero" – without lipid delivery being finally perfected and approved in 2018, there would have been no Covid-19 mRNA vaccines by 2020. Before Covid-19, there were many research studies looking at broader applications of combining this new lipid delivery technique with mRNA Dong says, including genetic disorders, cancer immunotherapy, infectious diseases and bacterial infections. "As long as you have the antigen and can sequence the protein, theoretically it should work". Thanks to the combined breakthrough in lipid delivery and mRNA technology, vaccines and treatments in development include Translate Bio's mRNA therapy's for cystic fibrosis and multiple sclerosis; Gritstone Oncology and Gilead Sciences' mRNA vaccine for HIV; Arcturus Therapeutics' therapies for cystic fibrosis and heart disease; and German start-up Ethris, with AstraZeneca, are developing mRNA therapies for severe pulmonary diseases and asthma. Solutions for tropical diseases are being explored too. Moderna are close to phase two (out of three) in clinical mRNA vaccine trials for both Zika and Chikungunya. Both are described as "neglected", so-called because they effect the poorest populations of the world and do not receive adequate research and funding. The speed and cost of mRNA vaccines could change that paradigm and signal the end of neglected tropical diseases. Perhaps the first new mRNA vaccine to hit our shelves, however, will be for a more familiar foe – the flu. Influenza viruses are responsible for an estimated 290,000–650,000 deaths annually worldwide. "We're most likely to see mRNA vaccines against influenza in the near future," says Whitehead. "These mRNA vaccines have been in development for years, and clinical trials to date have been encouraging. There are currently five clinical trials for Influenza A, including one in phase two". This could be just in time. Paul Hunter, a professor of health protection at the University of East Anglia in the UK who also consults for the WHO, has warned that some countries may be due an influenza epidemic that could lead to more fatalities than Covid-19. Several pharmaceutical companies are also pursuing mRNA vaccines and treatments for cancer. "Cancer cells will often have certain surface markers that the rest of the cells in your body don't have," says Blakney. “You can train your immune system to recognise and kill those cells, just like you can train your immune system to recognise and kill a virus: it's the same idea, you just figure out what proteins are on the surface of your tumour cells and use that as a vaccine". The idea of patient-specific, individualised medicine has been a tantalising prospect for years – this could be another door pushed wide open by mRNA, according to Blakney. In theory, "they take out your tumour, they sequence it, see what's on the surface of it, and then they make a vaccine specifically for you". If treatments for cancer, HIV and tropical disease are coming along with mRNA 2.0, then what could be even further down the line with 3.0? One huge area of concern for modern medicine is antibiotic resistance. "Potentially you could envision actually making a vaccine against a bacterial antigen such as C. difficile or some of those really tricky to treat bacteria," says Blakney. There are no trials yet, but scientific journals such as Frontiers have explored this idea. There's also potential for more general commercial health and wellbeing applications. For example, Fu suggests that lactose intolerance – that affects hundred of millions of people of Asian origin, including himself, and indeed an estimated 68% of the global population – could one day be targeted: "I'm missing the protein that allows me to break down lactose. In the future, you could develop some way of delivering the message, the mRNA, that that will make the protein that breaks down lactose… it's not life threatening, but I could imagine that being a billion-dollar industry." At Ohio State, Dong has even run a successful mouse trial targeting cholesterol. People with high levels of the protein PCSK9 tend to have high cholesterol and develop heart disease early. "We noticed that after one treatment [in mice], we can reduce the PCSK9 protein level by over 95%. That's definitely a very important research direction." At least one biotech company is planning a clinical trial using mRNA to inhibit PCSK9 according to Dong. You could take a whole bunch of different flavours… a cocktail of mRNAs that make different proteins selective for your particular need – Dragony Fu All this raises the question: could mRNA therapeutics give us almost superhuman immunity? Already Covid-19 mRNA vaccines lead some people to produce very high levels of antibodies, able to neutralise several variants of Covid-19 at once. There's also the potential to mix various mRNA vaccines together into a single health booster vaccine, which could ward off cancers and viruses at the same time. While it's just speculation at present, Fu says, "you could take a whole bunch of different flavours… a cocktail of mRNAs that make different proteins selective for your particular need." Both Moderna and Novavax already have combined Covid-19 and flu vaccines in development. Before we get too carried away, however, questions remain around mRNA vaccines. Currently we need regular booster shots – and these shots tend to hurt your arm, sometimes with fatiguing side effects. At the time of writing, we are less than a year into real-world use. Anaphylactic reactions (albeit with no deaths) have been observed in approximately 2 to 5 people per million vaccinated in the United States: slightly higher, 4.7 per million, with the Pfizer–BioNTech vaccine compared to 2.5 per million vaccinations from the Moderna vaccine. According to one analysis, while still low, this is 11 times higher than with the flu vaccine. "We're still working to understand how long the antibody response lasts for as well as the cellular response," says Blakney. "There's good indication now that you do get a really good memory T cell response from the mRNA vaccines, but since these trials are a year and a half old in most cases, we're still understanding how long that immunity lasts for." She adds that most people, "don't really want to get multiple vaccines every year that knock you out for three days afterwards". Blakney's lab at UBC is, however, working on an answer: saRNA, or self-amplifying mRNA. It has the same structural components as normal mRNA, except once inside a cell it can make copies of itself. "This is really advantageous because it allows you to use a much lower dose, usually about 100 times less saRNA compared to mRNA," says Blakney. This means more bang for your buck, and less pain in your arm. In a tortoise versus hare race, mRNA vaccines may have run ahead to combat Covid-19, but saRNA may win out in the end – and indeed has just received $195m (£145m) backing from AstraZeneca (which compares favourably to the $29.5m (£22m) Ethris received for its pulmonary diseases vaccine development, mentioned earlier in this article). Meanwhile, Fu, Dong, Whitehead and Blakney continue to ride – and drive – the wave of the RNAissance. Wherever it carries them, one thing is for sure: it will never again be the same niche, anonymous field of research they once knew. Especially if you put your explainer videos out on TikTok like Blakney. "My whole mission on there is to educate people about vaccines," she laughs. "I get tonnes of random questions. But I've also had loads of people say things like, you are the reason why me and my partner got the vaccine. And that's really impactful."
- 14 replies
-
- 3
-

-
.png)
-
- mrna
- superhuman
-
(and 2 more)
Tagged with:
-
- 391 replies
-
- 14
-

-
.png)
-

-
- vaccine
- vaccination
-
(and 2 more)
Tagged with:
-
https://asia.nikkei.com/Spotlight/Coronavirus/Pfizer-vaccine-94-effective-in-real-world-Israel-study?utm_campaign=RN Subscriber newsletter&utm_medium=coronavirus_newsletter&utm_source=NAR Newsletter&utm_content=article link&del_type=10&pub_date=20210225150000&seq_num=12&si=44594 Pfizer vaccine 94% effective in real world: Israel study Results from fast rollout help ease uncertainty in fight against COVID The research in Israel - two months into one of the world's fastest rollouts -- is providing a rich source of data outside of clinical trials. © Reuters February 25, 2021 10:35 JST JERUSALEM (Reuters) -- The first big real-world study of the Pfizer/BioNTech vaccine to be independently reviewed shows the shot is highly effective at preventing COVID-19, in a potentially landmark moment for countries desperate to end lockdowns and reopen economies. Up until now, most data on the efficacy of COVID-19 vaccines has come under controlled conditions in clinical trials, leaving an element of uncertainty over how results would translate into the real world with its unpredictable variables. The research in Israel - two months into one of the world's fastest rollouts, providing a rich source of data - showed two doses of the Pfizer shot cut symptomatic COVID-19 cases by 94% across all age groups, and severe illnesses by nearly as much. The study of about 1.2 million people also showed a single shot was 57% effective in protecting against symptomatic infections after two weeks, according to the data published and peer-reviewed in the New England Journal of Medicine on Wednesday. The results of the study for the Clalit Research Institute were close to those in clinical trials last year which found two doses were found to be 95% effective. "We were surprised because we expected that in the real-world setting, where cold chain is not maintained perfectly and the population is older and sicker, that you will not get as good results as you got in the controlled clinical trials," senior study author Ran Balicer told Reuters. "But we did and the vaccine worked as well in the real world." "We have shown the vaccine to be as effective in very different sub-groups, in the young and in the old in those with no co-morbidities and in those with few co-morbidities," he added. The study also suggests the vaccine, developed by U.S drugmaker Pfizer and Germany's BioNTech, is effective against the coronavirus variant first identified in the UK. Researchers said they could not provide a specific level of efficacy, but the variant was the dominant version of the virus in Israel at the time of the study. The research did not shed light on how the Pfizer shot will fare against another variant, now dominant in South Africa, that has been shown to reduce the efficacy of other vaccines. Of the nine million people in Israel, a nation with universal healthcare, nearly half have received a first dose, and a third have received both doses since the rollout began on Dec. 19. This made the country a prime location for a real-world study into the vaccine's ability to stem the pandemic, along with its advanced data capabilities. The study examined about 600,000 vaccinated people against the same sized control group of unvaccinated people. Researchers at Harvard T.H. Chan School of Public Health, Harvard Medical School and Boston Children's Hospital also collaborated. "This is more great news, confirming that the vaccine is around 90% effective at preventing documented infection of any degree of severity from 7 days after the second dose," said Peter English, a British government consultant in communicable disease control. "Previous recently studied papers from Israel were observational studies. This one used an experimental design known as a case-control study ... giving greater confidence that differences between the groups are due to their vaccination status, and not to some other factor." The study published on Wednesday was the first analysis of a national COVID-19 vaccination strategy to be peer-reviewed. It also offered a more detailed look at how the vaccine was faring at weekly intervals, while matching people who received the shot to unvaccinated individuals with similar medical histories, sex, age and geographical characteristics. Other research centres in Israel, including the Weizmann Institute of Science and the Israel Institute of Technology have shared several studies in recent weeks that show the vaccine to be effective. At least three studies out of Israel have also suggested the vaccine can reduce coronavirus transmission, but the researchers have cautioned that wider studies must be conducted in order to establish clear-cut conclusions. The Weizmann Institute's latest data shows a dramatic drop in illness - which began this month with the first age group vaccinated, the over-60s - has now extended to the two subsequent groups to have completed both doses. As infections have fallen in Israel, the country has eased its third national lockdown and reopened swathes of its economy including malls, shops, schools and many workplaces in the past two weeks. Recreational venues such as theatres, gyms and hotels opened on Sunday, but are open only to those deemed immune - holders of a "Green Pass", a health ministry document available for download only by people seven days after their second dose or people who have recovered from COVID-19. On Wednesday, Tel Aviv held one of the country's first live concerts after months of gatherings being banned under coronavirus restrictions. "This is so exciting, we are really so happy to be here today. It's unbelievable after one year of staying at home, it's great to be out to see some culture," said 60-year-old Gabi Shamir as she took her seat at the open-air show. Still, the vaccine's efficacy does not mean the country will be pandemic free any time soon. Like elsewhere in the world, a large proportion of the population are under 16 - about a third in Israel - meaning that they cannot yet get vaccinated as there have not been clinical trial results for children. "This is definitely not the end of the pandemic," said Eran Kopel, an epidemiologist at Tel Aviv University. "Once there is a safe vaccine for the children in Israel and all over the world we can then start to say that we could be approaching herd immunity."
-
this is really good news after covid ... the mRNA technology will be accelerated to be used on many other applications/vaccines .... spore huat ah!
-
I have a six month project that may need me to be stationed at Pfizer. Anyone knows the parking situation there? need to apply pass or anything?
-
Anyone been a labrat for them before? Me considering about going. Below are details I received from their email. They pay quit good and I really need money now. Anyone know any better way to make 3k in 2mths? ------------------------------------------------------------- Hello We are providing you with some study information to see if you or your friends are interested in participating in an upcoming research study at the Pfizer Clinical Research Unit (Singapore). If you or your friends wish to register or find out more about the study, please call the participant recruitment office at 6496-9888 or walk-in to PCRU, located at Level 10, Raffles Hospital. Vacancies for medical screening are limited and will be given on a first-come/call basis. Sincerely, Volunteer Recruitment Department Pfizer Clinical Research Unit (Singapore) www.PCRU.Pfizer.com Study A (Reimbursement for transportation & time inconvenience ~$4085) Males & Females aged 21- 55 years old Non , ex-smokers, smokers (<5 sticks/day) BMI : 17.5-30.5 kg/m2, Weighs more than 50kg Have not participated in other studies 30 days before study starts. No drug allergies -Consists of 4 periods: 4 days 3 nights stay, 2 outpatient visits plus 1 follow-up visit - Screening dates: Group 1: 11/8/08 (0800hrs or 12 noon) Group 2: 13/808 (12 noon) Group 1 Period 1: Admission (12noon): 20/8/08 (Wed) and stay till 23/8/08 (Sat) at 12 noon discharge Outpatient (Morning):24/8/08 (Sun) Outpatient (Morning):25/8/08 (Mon) Period 2: Admission (12noon): 27/8/08 (Wed) and stay till 30/8/08 (Sat) at 12 noon discharge Outpatient (Morning): 31/8/08 (Sun) Outpatient (Morning): 1/9/08 (Mon) Period 3: Admission (12noon): 7/9/08 (Sun) and stay till 10/9/08 (Wed) at 12 noon discharge Outpatient (Morning):11/9/08 (Thu) Outpatient (Morning):12/9/08 (Fri) Period 4: Admission (12noon): 14/9/08 (Sun) and stay till 17/9/08 (Wed) at 12 noon discharge Outpatient (Morning):18/9/08 (Thu) Outpatient (Morning):19/9/08 (Fri) Follow-up (4 Hrs): 22/9/08 (Mon)