Search the Community
Showing results for tags 'vaccine'.
-
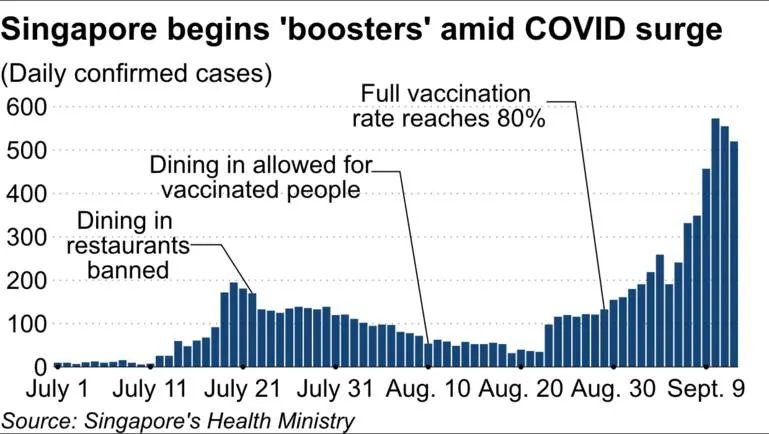
So now that we have reached a significant percentage milestone of vaccination, is it all green light now? Well, now comes the booster shot 🙂 Where to register: https://www.vaccine.gov.sg/ Why booster? https://www.usatoday.com/story/news/2021/09/13/covid-19-vaccine-booster-shots-who-can-get-them-when-still-uncertain/5773405001/ https://www.gov.sg/article/expanding-testing-and-surveillance-and-introducing-a-national-vaccine-booster-programme https://www.nature.com/articles/d41586-021-02158-6 Booster vaccination strategy MOH, with advice from the Expert Committee on COVID-19 Vaccination (EC19V), has reviewed the vaccination strategy in light of the more transmissible variants that have emerged globally. Although evidence globally and locally continues to show that vaccines are very effective in reducing severe illness and death, there is emerging data on the waning of vaccine efficacy against infection with time. MOH and EC19V will commence a booster programme for two groups of individuals: (A) Persons who are moderately to severely immunocompromised They are recommended to receive a third dose of the same Pandemic Special Access Route (PSAR) mRNA vaccine two months after their second dose as part of their primary course of vaccination regimen. They should be referred to do so by their doctors who have the best understanding of their medical condition. These include persons with the following conditions: Transplant patients on medications that suppress the immune system, including solid organ and allogenic stem cell transplants Cancer patients on active treatment with chemotherapy or on other therapies that suppress the immune system Haematological cancers Treatments for non-cancer conditions that suppress the immune system End-stage kidney disease on dialysis Advanced or untreated HIV (B) Persons aged 60 years and above and residents of aged care facilities They are recommended to receive a booster dose of PSAR mRNA vaccine six to nine months after having completed their primary course of vaccination regimen. The first batch of seniors aged 60 years and above completed their second doses around March 2021 and therefore will be eligible for the third dose within the month of September. More details on the implementation of the booster shot will be announced later. Not this type of booster ya https://asia.nikkei.com/Spotlight/Coronavirus/COVID-vaccines/Singapore-starts-booster-shots-as-COVID-cases-hit-1-year-high
-
https://www.todayonline.com/singapore/adulting-101-untimely-deaths-friends-existential-crisis-2131826 Adulthood is an invigorating stage of life as young people join the workforce, take on more responsibilities and set their sights on the future. But its many facets — from managing finances and buying a home to achieving work-life balance — can be overwhelming. In this series, TODAY’s journalists help young Singaporeans navigate this stage of their lives and learn something themselves in the process. SINGAPORE — In the last two years, five people I knew died from sudden cardiac arrest. They were young and seemingly healthy people whose untimely demise came as a shock to their family and friends. They ranged in age from about 25 to 35. The grief hit me pretty hard, as I felt a lot of guilt and regret about my relationship with some of them. It got me to thinking — are more young people dying from sudden cardiac arrest? And should I be worried? Cardiologists from the National University Heart Centre Singapore told me that the risk of sudden deaths in young people remains exceedingly low. Based on the out-of-hospital cardiac arrest (OHCA) data report published by the Singapore Heart Foundation four years ago, those above the age of 65 constitute the highest risk group of patients, accounting for 36.2 per cent of the 3,000 cases of cardiac arrest in 2019. There are about 3,000 cases of OHCA yearly here. One cardiologist told me that the prevalence of OHCA in Indians and Malays is twice the rate of Chinese. The prevalence of OHCA in men is also twice that of women. While uncommon, there are underlying conditions among young adults that can lead to sudden deaths, such as hypertrophic cardiomypathy (abnormal thickening of heart muscles) or arrhythmias (abnormal heart rhythm of genetic causes), another cardiologist said. This is why health screenings are important, as people often believe themselves to be healthy if they do not have any symptoms of underlying disease when they may have conditions that are asymptomatic. Though sudden cardiac arrest among young people with no underlying conditions is rare, I could not shake this feeling that my life could be taken from me at any time. This led me to move beyond concerns over cardiac arrest affecting the young to wonder more broadly at the meaning of my brief, mortal existence. I was left feeling unmotivated and uneasy. Don’t get me wrong, my life is going okay — I have a good job, I have a roof over my head, I have two beautiful kids and a supportive husband — but I was being consumed by this thought that if life is so short, why bother doing anything? It occurred to me that I was perhaps having an existential crisis. Mr Praveen Nair, a psychologist at Raven Counselling and Consultancy, said that this occurs when there is an inner conflict within an individual causing them to break from traditional thinking patterns and recalibrate to become more contemplative with regard to questions about meaning, purpose and identity in life. Mr Nair also reassured me that I am not the only one feeling this way, as he has seen more adult clients with similar issues. One contributor to this is social media, said Mr Nair, as some netizens cherry pick what they share online to present a rosy picture of their lives, which can cause other users viewing the content to experience "fomo" (fear of missing out). This, in turn, can lead them to wondering about their direction in life. Mr Nair said it is normal to experience an existential crisis even when things in your life seem to be going okay as many things, both overt and subliminal, can influence our thoughts even when our lives are relatively routine. In fact, some argue that the mundane and routine can be a stimulus to initiating thoughts about the larger meaning or purpose of life. This makes sense, as I have been feeling a certain kind of tedium for some time now, juggling work and caring for two young children daily. Ms Abigail Yang, a grief therapist at counselling platform Talk Your Heart Out, said that it is normal to think deeply about life or question how you feel about it. The more fundamental issue is when no answer satisfies you, she added. “It becomes a constant loop of complex questions with no fulfilling solution. This, in turn, leads to a conflict within yourself about your reason for existence,” said Ms Yang. In some cases, extreme thoughts and unanswerable questions can leave one feeling frustrated, anxious, depressed and even suicidal, said experts. They also shared that one way to overcome an existential crisis is to disengage from pursuits or people that bring me no joy and redirect my energy to those that do. Mr Nair said this can help initiate renewed drive and motivation in life. “It may sound counterintuitive but many great innovations occurred when inventors experienced an existential crisis. They channelled their energies into a new venture that was motivating,” he told me. One way to overcome the crisis is to also take time to connect more with people whose company I enjoy, as an existential crisis can occur when we feel disconnected from others, said Mr Nair. Ms Yang reminded me that it is okay to allow myself to feel such negative emotions, and that I should not suppress them. Some people block out pain and suffering, thinking this will make them happy, but it can often lead to a false sense of happiness, she said. Embodying emotions and acknowledging feelings of pain, discontentment and dissatisfaction can open the door to personal growth, and improve one’s outlook on life, Ms Yang added. One tip I got from a friend that has helped me deal with my existential crisis is this: "KonMari" your schedule, rid yourself of self-imposed duties and obligations and identify areas where you could be doing less, doing something easier, or doing nothing at all. This is a reference to Japanese author Marie Kondo and her ideas about ridding our lives of needless clutter. This might mean, for instance, your one-hour exercise routine becomes a 20-minute one, or perhaps you ditch it all together for an extra hour of sleep. The only person that should be happy with the choice you make is you. ABOUT THE WRITER: Nabilah Awang, 29, is a former Senior Journalist at TODAY.
- 101 replies
-
- 1
-

-
better late than never...probably stop taking the same old booster. this one effective against delta and omicron. https://abcnews.go.com/US/moderna-booster-fall-turning-point-covid-fight/story?id=85254442 Moderna says new booster for fall could be 'turning point' in COVID fight With vaccine immunity waning, and concerns over a fall surge growing, officials from Moderna announced on Wednesday that data from its study on Omicron-containing bivalent booster, revealed that it offers superior antibody response against omicron – one month after injection - compared to the company's current vaccine. Moderna Chief Medical Officer Dr. Paul Burton told ABC News that he believes the company's "highly effective" updated COVID-19 bivalent vaccine could be a "turning point" in the nation's fight against the pandemic. "The data are definitely better than I had even hoped," Burton told ABC News that in an interview. "Given the magnitude of effect — that seven-fold increase in antibody levels — we could for the first time, be at a vaccine that is truly effective with once yearly dosing because we know those antibody levels will decay." Moderna plans to file its data with the Food and Drug Administration "as quickly as possible," and should the vaccine be authorized, the company will be ready to supply the shots to "as many people around the world as possible," Burton said. "These are very important data. It's an important announcement. And I think it has the potential to be a real turning point in this latter part second half of the pandemic," Burton said. The announcement comes as the U.S. continues to battle a secondary surge from omicron subvariants amid waning immunity, relaxed attitudes by many towards mitigation measures and fatigue and skepticism about vaccination and booster shots. After more than a year of persistent efforts and messaging from federal and local authorities, just under 71% of the eligible population aged 5 and over is fully vaccinated and less than half who have completed their primary series have had their first booster dose. Burton said the company hopes this new vaccine could, for the first time, provide a roadmap for annual COVID-19 vaccinations, rather than shots every few months. "I believe that we will be able to get to this once yearly dosing now because we have high levels now. That will probably even increase and mature over time, potentially giving people protection over a full year. We could finally get to that once yearly protection, so I think it's really important," Burton said. The variant adapted vaccine, which contains omicron mRNA, was found to be highly effective against omicron, Burton said. The company reported that this new bivalent vaccine combines the original shot and the omicron mRNA together in a single shot. "The original vaccine gives great protection against delta and other variants that we've seen still recently, and that really caused significant disease. Omicron does cause significant disease. It's definitely not mild, but it's super infectious. You need to combine the two together," Burton explained. Although it is still unknown how well the new vaccine will be able to prevent infections and severe illness, Burton said he is confident that the vaccine will "definitely prevent hospitalization and death." "It's highly effective," Burton said, adding that the safety profile is also "very robust," and "reassuring." Should another variant of concern emerge, one drastically different than omicron and the already existing variants, scientists at Moderna will be prepared to reevaluate and readapt the shot to address the new threat. "If something again really drastic occurs, Well, we'll have to look at that. But I would say again, what I think we've been able to show here, is that we can adapt very quickly. We were able to make this new booster in weeks and get it into testing. I think we can even speed that up now. So, I think we were well prepared for that," Burton said. [9:45 in] Burton said he remains worried about the growing population of "unprotected" and "under-vaccinated" Americans, who will be vulnerable to more severe disease. "But by the fall, here, and around the world, we're gonna have many people who are unvaccinated and under- vaccinated and they're going to be very vulnerable," Burton said. "By Autumn, we know that waning occurs. Even people who got boosted in the new year, come the autumn of this year, they're going to have low levels of antibody. They're going to be unprotected, under-vaccinated and they're going to be very vulnerable." The sentiment of booster fatigue among some Americans is another concern for Burton, he said. "The fatigue and just the eagerness of all of us... to kind of put COVID behind us has led to apathy and a real reduction in boosting," Burton said. As Moderna also prepares for the FDA to consider authorizing its pediatric vaccine for children under six years-old, Burton said he hopes the youngest Americans will also have access to this updated shot as well, in the fall, once they receive their primary series. "I think the issue for the littlest kids is that they have no protection right now, they have had no primary vaccination they're under protected," Burton said. "I would propose that parents, caregivers get their little kids vaccinated now, They'll be ready to go back to school with Moderna. We know that two doses will give them protection... I think it's likely that they will need an additional boost at some point. And I would propose, and I think we all would imagine, that this is then the booster for them to get in the autumn of this coming year." The company is conducting studies to look into safety and efficacy of the shots in young children, and scientists are expected to have data later in the year, Burton said.
-
Singapore passports to be valid for 10 years for applications from October https://www.channelnewsasia.com/news/singapore/singapore-passport-validity-extended-10-years-renewal-ica-14760828
-
Singapore may get non-mRNA Novavax Covid-19 vaccine before year-end SINGAPORE - Singapore has signed advanced purchase agreements with American biotechnology company Novavax to secure its protein-based Covid-19 vaccine, with supplies possibly arriving before the end of the year. Singapore's Ministry of Health (MOH) had signed the agreements with Novavax in January this year, Health Minister Ong Ye Kung said on Thursday (June 24) at a virtual press confernce held by the multi-ministry task force on Covid-19. He added that MOH has been looking for vaccines of good quality that are safe and effective, to be part of the national vaccination programme, and noted that Novavax has recently shown encouraging results. Currently only the Pfizer-BioNTech and Moderna vaccines, which use the mRNA (messenger ribonucleic acid) technology, are offered under the national vaccination programme. These vaccines teach cells to make a protein that prompts an immune response. Novavax, a protein-based vaccine, uses a laboratory-made version of the Sars-CoV-2 spike protein to stimulate an immune response. Like the Pfizer-BioNTech and Moderna vaccines, it also requires two shots spaced apart. Mr Ong said shipments of Novavax's vaccines will not be ready so soon as it is still undergoing phase three clinical trials. "In the meantime, please consider mRNA vaccines, they work very well," he added. Novavax has been shown to be more than 90 per cent effective against a variety of Covid-19 variants, based on late-stage data from its clinical trial in the United States. The study of nearly 30,000 volunteers in the US and Mexico puts Novavax on track to file for emergency authorisation in the US and elsewhere in the third quarter of the year, the company said on June 14. The company added that its vaccine candidate was more than 93 per cent effective against the predominant variants of Covid-19 that have been of concern among scientists and public health officials. Protein-based vaccines are a conventional approach that uses purified pieces of the virus to spur an immune response. Vaccines against whooping cough and shingles employ this approach.
-
Good news for the world. But pfizer and moderna are disappointing. mRNA supposed to be fast to develop, but now Apr, apparently still nothing, although they promised omicron-specific vaccine in Mar. Omicron-specific Sinopharm, Sinovac COVID vaccine candidates cleared for clinical trial BEIJING, April 16 (Reuters) - COVID-19 vaccine candidates developed by a Sinopharm subsidiary and Sinovac Biotech (SVA.O) to target the Omicron variant were approved for clinical trials in Hong Kong, the companies said on Saturday. Scientists worldwide are racing to study upgraded injections against Omicron, as data indicated that antibodies elicited by vaccines based on older strains show weaker activity to neutralise the highly transmissible variant. The two candidates from units of Sinopharm subsidiary China National Biotec Group (CNBG) and one from Sinovac contain inactivated or "killed" coronavirus and are similar to vaccines that the companies are supplying in China and overseas, the companies said in statements. The Sinopharm candidates will be tested as boosters in adults who have already received two or three vaccine doses, CNBG said. It did not specify which vaccine products the trial participants would have received before taking the experimental booster, or how many subjects would be recruited. Sinovac said it will push forward studies in its existing CoronaVac vaccine's protection against emerging variants. A Chinese study showed that a fourth dose of BBIBP-CorV, an existing Sinopharm COVID vaccine, did not significantly lift antibody levels against Omicron when administered six months after a third booster dose to a regular two-dose regimen. While the fourth dose restored antibody levels to around the peaks that followed the third dose, researchers said new vaccines would offer a better alternative as future boosters. Reporting by Roxanne Liu and Andrew Galbraith; Editing by Edmund Klamann and William Mallard
-
https://asia.nikkei.com/Spotlight/Coronavirus/COVID-vaccines/China-s-quest-for-mRNA-vaccine-hits-stumbling-block-in-omicron?utm_campaign=GL_coronavirus_latest&utm_medium=email&utm_source=NA_newsletter&utm_content=article_link&del_type=10&pub_date=20220311150000&seq_num=8&si=44594 China's quest for mRNA vaccine hits stumbling block in omicron Limited effectiveness of conventional shots puts 'zero-COVID' policy at risk A medical worker walks past a sign at a coronavirus vaccination site in Beijing. © Reuters SHIN WATANABE, Nikkei staff writerMarch 10, 2022 12:01 JST DALIAN, China -- China continues to struggle in developing a homegrown mRNA vaccine against COVID-19, with its front-runner failing to reach the market by the end of last year as hoped and now showing disappointing results against the highly infectious omicron variant. The messenger RNA-based ARCoV vaccine candidate developed by Abogen Biosciences, Walvax Biotechnology and the Academy of Military Medical Sciences, now in Phase 3 clinical trials, encountered a snag shown in a paper published last month in the journal Cell Research. Eight of 11 participants in a Phase 1 early trial who received two doses of ARCoV showed "low" neutralizing antibody activity against omicron. Though the study found that a third dose significantly increased antibody levels in mice, the shot's efficacy against the variant remains in question. Walvax Vice Chairman Huang Zhen had boasted to Chinese media in July that the vaccine would be out by year-end. But trials continue, and no time frame exists for a release. China's stumbles in this area could put further strain on the government's strict "zero-COVID" policy as the omicron variant drives a resurgence in cases. The political risk consultancy Eurasia Group in January cited "vaccines with limited effectiveness" as a threat to China's coronavirus strategy. Chinese state-owned pharmaceutical companies moved quickly on conventional vaccines against COVID-19, rolling out two in early 2021. Both are inactivated vaccines, which use "killed" viruses that cannot infect cells but still can cause an immune response. Unlike the mRNA vaccines from Pfizer and Moderna, these shots do not need to be kept at ultracold temperatures, enabling China to export them far and wide. But these inoculations have proven less effective than their mRNA counterparts, eroding trust in Chinese-made shots. A growing number of countries that initially used Chinese vaccines, such as Indonesia, are turning to the U.S. for boosters. The Pfizer and Moderna inoculations went on the market in late 2020, and are currently being tested against the omicron variant. Developing mRNA vaccines is no easy task, said Shi Jinjun, an associate professor at Harvard Medical School. Shi said the companies that succeeded have more than a decade of research behind them, and so were well prepared when the coronavirus emerged. Abogen was established in 2019 by Ying Bo, a former Moderna scientist. The company raised more than $1 billion through November from sources including the SoftBank Vision Fund. But while investors have high hopes for Abogen, it is unclear whether such a young company can meet these expectations. Sinopharm, one of China's early COVID-19 vaccine creators, broke ground in September on an mRNA vaccine production facility in Shanghai slated to be completed in September of this year, according to local media. Researchers face heavy pressure to advance mRNA shots to mass production and dispel skepticism of Chinese vaccines.
-
https://www.bloomberg.com/news/articles/2021-05-28/singapore-vaccine-plan-students-then-open-season-balakrishnan Singapore Plans to Vaccinate Students First, Then Everyone Else Singapore plans to roll out vaccines to students, followed by everyone else eligible, in what will be a “great acceleration” of vaccinations in the country, Foreign Minister Vivian Balakrishnan said in an interview on CNN. “Our next step is that we’re going to offer vaccination to our school students, the teenagers,” Balakrishnan said, “following which it’ll be open season for everyone in Singapore.” The city-state earlier flagged that almost all of its eligible population could be given at least the first dose of a Covid-19 vaccine by the end of August. Vaccinations in Singapore are currently open to those aged 40 and above, as well as for priority workforces, such as first responders, hospital staff and airport workers. About 37% of Singapore’s population has had its first jab of the vaccine, a rate far ahead of most developed Asian economies, on par with the European Union’s average, but trailing the U.S., U.K. and Middle Eastern financial hubs like Israel, the U.A.E. and Qatar. Singapore is one of several countries, mostly in Asia, that have largely kept Covid caseloads and deaths to a minimum but have struggled to reopen. The city-state has tightened its borders and, following a recent uptick in cases, suspended in-person dining, transitioned most schools to at-home learning, and limited groups to a maximum of just two. Widespread vaccination, then, has become Singapore’s key to reopening. Balakrishnan said the country has not seen significant vaccine hesitancy, though has been limited by supply. However, infrastructure that can support additional injections capacity is in place, and the country aims to double its vaccination pace. “Watch this space,” he said. “You’re going to see a great acceleration.”
-
The Pfizer vaccine showed efficacy of 95% The Moderna vaccine was 94.1% Sinovac is 50.4%, dafug! https://asia.nikkei.com/Spotlight/Coronavirus/Sinovac-vaccine-disappoints-at-50.4-efficacy-latest-Brazil-data Sinovac vaccine disappoints at 50.4% efficacy: latest Brazil data Results come as scientists slam biomed center for raising 'unrealistic' hopes The Butantan biomedical production center in Sao Paulo, Brazil, is being blasted by scientists and observers for releasing partial data only days ago that generated unrealistic expectations. © Reuters January 13, 2021 10:34 JST SAO PAULO (Reuters) -- A coronavirus vaccine developed by China's Sinovac Biotech was just 50.4% effective at preventing symptomatic infections in a Brazilian trial, researchers said on Tuesday, barely enough for regulatory approval and well below the rate announced last week. The latest results are a major disappointment for Brazil, as the Chinese vaccine is one of two that the federal government has lined up to begin immunization during the second wave of the world's second-deadliest COVID-19 outbreak. Several scientists and observers blasted the Butantan biomedical center for releasing partial data just days ago that generated unrealistic expectations. The confusion may add to skepticism in Brazil about the Chinese vaccine, which President Jair Bolsonaro has criticized, questioning its "origins." "We have a good vaccine. Not the best vaccine in the world. Not the ideal vaccine," said microbiologist Natalia Pasternak, criticizing Butantan's triumphant tone. Last week, the Brazilian researchers had celebrated results showing 78% efficacy against "mild-to-severe" COVID-19 cases, a rate they later described as "clinical efficacy." They said nothing at the time about another group of "very mild" infections among those who received the vaccine that did not require clinical assistance. Ricardo Palacios, medical director for clinical research at Butantan, said on Tuesday that the new lower efficacy finding included data on those "very mild" cases. "We need better communicators," said Gonzalo Vecina Neto, a professor of public health at the University of Sao Paulo and former head of Brazilian health regulator Anvisa. Piecemeal disclosures about Chinese vaccine trials globally have raised concerns that they are not subject to the same public scrutiny as U.S. and European alternatives. Palacios and officials in the Sao Paulo state government, which funds Butantan, emphasized the good news that none of the volunteers inoculated with CoronaVac had to be hospitalized with COVID-19 symptoms. Public health experts said that alone will be a relief for Brazilian hospitals that are buckling under the strain of surging case loads. However, it will take longer to curb the pandemic with a vaccine that allows so many mild cases. "It's a vaccine that will start the process of overcoming the pandemic," Pasternak said. Researchers at Butantan delayed announcement of their results three times, blaming a confidentiality clause in a contract with Sinovac. In the meantime, Turkish researchers said last month that CoronaVac was 91.25% effective based on an interim analysis. Indonesia gave the vaccine emergency use approval on Monday based on interim data showing it is 65% effective. Butantan officials said the design of the Brazilian study, focusing on frontline health workers during a severe outbreak in Brazil and including elderly volunteers, made it impossible to compare the results directly with other trials or vaccines. Still, COVID-19 vaccines in use from Pfizer Inc with partner BioNTech SE and Moderna Inc proved to be about 95% effective in preventing illness in their pivotal late-state trials. The disappointing CoronaVac data is the latest setback for vaccination efforts in Brazil, where more than 200,000 people have died since the outbreak began - the worst death toll outside the United States. Brazil's national immunization program currently relies on CoronaVac and the vaccine developed by Oxford University and AstraZeneca Plc - neither of which has received regulatory approval in Brazil. Anvisa, which has stipulated an efficacy rate of at least 50% for vaccines in the pandemic, has already pressed Butantan for more details of its study, after it filed for emergency use authorization on Friday. The regulator said it will meet on Sunday to decide on emergency use requests for CoronaVac and the British vaccine. AstraZeneca failed to deliver active ingredients to Brazil over the weekend, leaving the government scrambling to import finished doses of the vaccine from India to begin inoculations.
-
Valneva says early studies show COVID-19 vaccine effective against Omicron Jan 19 (Reuters) - French biotech firm Valneva (VLS.PA) said on Wednesday that preliminary studies showed that three doses of its inactivated COVID-19 vaccine candidate neutralised the Omicron variant of the disease. All of the serum samples tested presented neutralizing antibodies against the ancestral virus and Delta variant, it said, while 87% of samples did so against the Omicron variant. "We are extremely pleased with these results," said Chief Medical Officer Juan Carlos Jaramillo in a statement, noting that these added to an earlier Phase III trial that showed improved immune response with two doses of the VLA2001 candidate. Valneva expects to receive potential approvals for its vaccine within the first three months of 2022, and is providing data to the European Medicines Agency (EMA) as well as regulators in the UK and Bahrain.
-
https://www.ndtv.com/world-news/norway-warns-of-vaccination-risks-for-sick-elderly-patients-after-23-die-2353257 Norway Warns of Vaccination Risks For Sick Elderly Patients After 23 Die Norwegian officials said 23 people had died in the country a short time after receiving their first dose of the vaccine. Of those deaths, 13 have been autopsied, with the results suggesting that common side effects may have contributed to severe reactions in frail, elderly people, according to the Norwegian Medicines Agency. Pfizer and BioNTech are working with the Norwegian regulator to investigate the deaths in Norway, Pfizer said in an e-mailed statement. The agency found that "the number of incidents so far is not alarming, and in line with expectations," Pfizer said. Allergic reactions have been uncommon so far. In the U.S., authorities reported 21 cases of severe allergic reactions from Dec. 14-23 after administration of about 1.9 million initial doses of the vaccine developed by Pfizer Inc. and BioNTech SE. That's an incidence of 11.1 cases per million doses, according to the Centers for Disease Control and Prevention. Though both Covid-19 vaccines approved so far in Europe were tested in tens of thousands of people -- including volunteers in their late 80s and 90s -- the average trial participant was in his or her early 50s. The first people to be immunized in many places have been older than that as countries rush to inoculate nursing-home residents at high risk from the virus.
-
With a high efficacy close to mRNA, it should be good news to poorer countries. Texas scientists’ new Covid-19 vaccine is cheaper, easier to make and patent-free Dr Maria Bottazzi says their vaccine, called Corbevax, is unique because they do not intend to patent it Erum Salam Sat 15 Jan 2022 10.00 GMT A new Covid-19 vaccine is being developed by Texas scientists using a decades-old conventional method that will make the production and distribution cheaper and more accessible for countries most affected by the pandemic and where new variants are likely to originate due to low inoculation rates. The team, led by Drs Peter Hotez and Maria Bottazzi from the Texas Children’s Hospital Center for Vaccine Development at Baylor College of Medicine, has been developing vaccine prototypes for Sars and Mers since 2011, which they reconstructed to create the new Covid vaccine, dubbed Corbevax, or “the world’s Covid-19 vaccine”. Although more than 60 other vaccines are in development using the same technology, Bottazzi said their vaccine is unique because they do not intend to patent it, allowing anyone with the capacity to reproduce it. “Pretty much anybody that can make hepatitis B vaccines or has the capacity to produce microbial-based protein like bacteria or yeast, can replicate what we do,” Bottazzi said. Patent wars over mRNA vaccines have recently heated up. Moderna and the National Institutes of Health are in a dispute over who should get credit for specific discoveries that led to a Covid-19 vaccine which has been delivered to more than 73 million Americans. If Moderna is found to have infringed on the federal government’s patent, it could be forced to pay more than $1bn. At the same time, activists have called for Pfizer and Moderna to share the technology and knowhow for producing their vaccines, including taking the fight to the World Trade Organization. Low-income countries, which have few vaccine research and production facilities, have vaccinated just one in nine people, according to the World Health Organization. The US has fully vaccinated 67% of the population and provided a third vaccine dose to more than one-third. Corbevax’s clinical trial data has yet to be released due to resource constraints, but Texas Children’s hospital said the vaccine was over 90% effective against the original Covid-19 strain and over 80% effective against the Delta variant. The vaccine’s efficacy against the Omicron variant is currently being tested. The process to create the vaccine involves the use of yeast – the same method by which hepatitis B vaccines are produced. The Moderna, Pfizer and Johnson & Johnson vaccines currently authorized in the US use different technologies, or vaccine “platforms”. Moderna and Pfizer use messenger RNA (mRNA) technology. This platform introduces the immune system to Covid-19 by delivering instructions on how to produce its most recognizable feature, the spike proteins which coat its surface. This helps the immune system recognize and fight the virus later, if a person is exposed. Johnson & Johnson’s vaccine introduces immune cells to the spike protein through an otherwise harmless cold virus, a technology called viral vector. The Corbevax vaccine uses a platform called recombinant protein sub-unit technology, which places an actual piece of Covid-19’s spike protein in yeast cells. The yeast cells then copy the vital protein and the protein is introduced to the immune system. “We make the protein, directly and synthetically, in the lab using the yeast system,” Bottazzi explained. “We ask the yeast to make a protein that looks just like a protein that is made by the virus. Then we immunize the protein and the body then processes this protein and presents it to the immune system. Therefore, you don’t ask your body to do any major manipulation of the coding.” Crucially, storing the Corbevax vaccine only requires standard refrigeration, unlike the Pfizer vaccine, which requires ultra-cold storage in transit. Biological E, an Indian pharmaceutical company accustomed to producing hepatitis B vaccines with whom Bottazzi’s team has a longstanding relationship, has already produced 150m doses of the new Corbevax vaccine and will soon be able to produce 100m doses every month. After being overlooked by government organizations for funding, Bottazzi said, the developers behind Corbevax relied on philanthropic donations to get them over the finish line. The Texas Children’s Hospital Center for Vaccine Development is an academic and scientific institution in nature, but Bottazzi said developing Corbevax had forced them to stretch their resources in order to gain visibility as a serious candidate for Covid vaccine development. “We ourselves are learning how to do work that is regulatory-enabling, that enables good quality, good reproduction, good record-keeping … we mimic as if we were a small biotech or manufacturing entity,” she said. “Every technology has pros and cons. Nobody is claiming one is the super-duper, only solution. All the [vaccines] are part of the solution. But when you have a situation of such gravity around the world, you don’t pick and choose a solution – you try to use all solutions,” Bottazzi said. Bottazzi said the reason she and her team did not patent the vaccine was because of her team’s shared philosophy of humanitarianism and to engage in collaboration with the wider scientific community. “We want to do good in the world. This was the right thing to do and this is what we morally had to do. We didn’t even blink. We didn’t think, ‘how can we take advantage of this?’ You see now that if more like us would have been more attuned to how the world is so inequitable and how we could have helped from the beginning so many places around the world without thinking ‘what’s going to be in it for me?’, we could have basically not even seen these variants arise.” Bottazzi hopes her move will incentivize others to follow suit and make affordable and accessible vaccines for other diseases and viruses, like hookworm. “We need to break these paradigms that it’s only driven by economic impact factors or return of economic investment. We have to look at the return in public health.”
- 7 replies
-
- 5
-

-
- texas
- scientists
-
(and 3 more)
Tagged with:
-
Sinovac lai liao. I guess HSA will fast track approve them for use, no point keeping them in cold storage space. Just in in time for the general population starting in April. Keeping finger crossed not to get it. https://www.moh.gov.sg/news-highlights/details/1-new-case-of-locally-transmitted-covid-19-infection-24-Feb-2021-Updated
- 394 replies
-
- 5
-

-
- healthcare and wellness
- covid-19
-
(and 3 more)
Tagged with:
-
Last month I watched Steven's video Travelling during a pandemic and didn't think much of it. Firstly, because he's from the media so kinda duty-bound to travel. Secondly, no other "famous people" utilising the VTL. Thirdly, no one around me talking about it either so it's not relatable at all. I watched his video in envy though. Fast-forward a month later, Shane Pow, who was recently released from prison after having served his jail time for drink driving and signed on with Lee Nan Xing's talent management agency, is going to Germany as well for work! https://www.asiaone.com/entertainment/shane-pow-going-germany-pornsak-livestream-and-sell-things-vtl-mdada An influencer whom I follow has also just booked her trip via SIA to Europe for February 2022! And today, I got to know that two of my colleagues will be going on a trip to Paris next month for a short holiday! @Ragingbull And another will be going on a press trip to Europe as well. Just checked SIA and the cost is about 1K to Europe. Not sure how to book the VTL flights though. I don't think I'll be traveling anytime soon, at least not this year but I'm excited that the travel bug is back and gaining traction. It's only a matter of time before the rest of us overcome our post-pandemic travel anxiety and start seeing the world again. One major concern of mine other than COVID-19 is of course racism in Europe. Btw, this thread is for everyone who intends to move on from this pandemic. So the kpkb ones, don't la. There are other threads for you to scold the government ok? 💕 I miss the MCFers travel threads! @Ragingbull you know what to do!
-
Plug-and-play vaccine, a good read: Prof Sarah Gilbert, Covid vaccine creator: Now let’s take on 12 more diseases If you head to the cutting edge of vaccinology you will find Prof Dame Sarah Gilbert, from the Jenner Institute and the architect of the Oxford vaccine. Using a revolutionary technology, the team at Oxford had a vaccine ready to start clinical trials in just 65 days. In partnership with pharma giant AstraZeneca, more than 1.5 billion doses have been distributed around the world. The new generation of vaccines are quick to make and highly flexible. "It's like decorating a cake," says Prof Gilbert. The old-school method of developing vaccines means you must go back to the raw materials and start from scratch for every vaccine you make. It is like starting with a bench of flour, sugar, eggs and butter. The next step is to take the offending virus, or other disease-causing microbes, and either kill it or weaken it to make a vaccine. Take the two seasonal flu vaccines that are given each year. The adult jab is made by growing influenza viruses inside eggs. The viruses are then purified and killed to make the vaccine. The nasal spray for children has live viruses, but these are made weak and unstable so they can grow in the cooler temperatures of the nose, but not in the warmth of the lungs. But it takes a lot of work to start from scratch for every new disease and there is plenty that can go wrong. You can end up with the vaccine-equivalent of a soggy bottom. The development of Oxford's coronavirus vaccine used a completely different approach known as "plug-and-play". With this type of vaccine most of the work has already been done - the cake has been pre-baked, it just needs to be "decorated" in order to match its target. "We've got the cake and we can put a cherry on top, or we can put some pistachios on top if we want a different vaccine, we just add the last bit and then we're ready to go," Prof Gilbert tells Inside Health. Two of the other big Covid vaccines - one made by Pfizer-BioNTech and the other by Moderna - use another style of highly adaptable plug-and-play vaccine technology. And all these technologies should make it quicker and easier to develop the vaccines of the future. "There's a lot of vaccine development that we need to do now that we can do it," says Prof Gilbert. Top of her list of targets are the official "priority pathogens". While Covid was a surprise, these are the known threats that are bubbling away with the potential to cause large outbreaks and potentially the pandemics of the future. Some of this work is already under way. Oxford has started clinical trials of a plague vaccine using its plug-and-play technology. Plague infamously caused the Black Death pandemic killing hundreds of millions of people. Separately Moderna is already looking at using its own mRNA technology to make a Nipah vaccine. The virus kills up to three-quarters of infected people. Yet, the big barrier for tackling these diseases will be the same as it has always been - money. They affect some of the poorest parts of the world and there is concern that, even in the wake of pandemic, research won't be funded. And, while vaccine technology has leapt forward - the old enemies are still the same and some have tricksy quirks that mean they pose monumental challenges. All vaccines need a target - called an antigen - that they train the immune system to attack. For all the problems Covid has caused, the virus was a pretty simple beast and the target antigen was blatantly obvious. The outer surface of the virus is covered in spike proteins. So all researchers had to do was plug in the genetic blueprints for the spike protein, train the body to recognise it and be pretty confident that the vaccine was going to work. However, the target antigen is not obvious in other more complex microbes such as the three big killers - malaria, HIV and tuberculosis. HIV is a constantly moving target. It is a shape-shifter that rapidly mutates in order to alter its appearance and outwit our immune system. It is hard to know how to pin it down. We already have vaccines against malaria and tuberculosis, but they are far from perfect. Read more : https://www.bbc.com/news/health-58898085
-
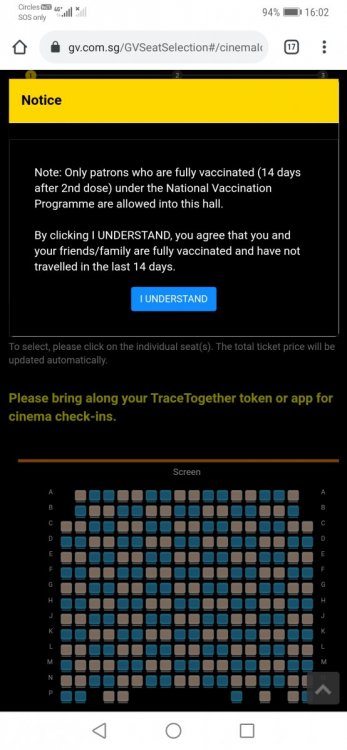
Saw this commentary article on CNA with an interesting question. We all know that anti-vaccination movements in western countries like the US is pretty strong but in sg I feel that its not as prominent?? Are there actually people in sg that are strongly against vaccination? Not sure if its because of gov censorship or Singaporeans generally have a lack of opinion on this matter. So it comes back down to the commentary question: Will you unfriend someone because they refuse to get vaccinated? 😂 Gov also making gatherings with friends harder with the whole only vaccinated people get to dine-in rule and to constantly accommodate that one friend who is unvaccinated may get annoying after a while? Share some stories leh.
- 93 replies
-
- 1
-

-
- 387 replies
-
- 14
-

-
.png)
-

-
- vaccine
- vaccination
-
(and 2 more)
Tagged with:
-
Book a Toyota now and walk away with $560 worth of petrol vouchers if you're fully vaccinated! 😆 https://www.sgcarmart.com/new_cars/newcars_adpage.php?Make=Toyota
- 20 replies
-
- 16
-

-
.png)
-

-

-
- toyota
- vaccinated
- (and 4 more)
-
BioNTech vaccine creates 10 times more antibodies than Sinovac, study finds https://www.scmp.com/news/hong-kong/health-environment/article/3141285/coronavirus-hong-kong-biontech-vaccine-recipients "Though quantity of coronavirus-targeting proteins does not directly correlate to level of immunity, findings may suggest ‘substantial differences in vaccine effectiveness’ But study’s lead author notes moderate levels of protection are better than none at all, saying: ‘Don’t let the perfect be the enemy of the good’ ."
-
https://asia.nikkei.com/Spotlight/Coronavirus/Japan-nears-homegrown-vaccine-with-Daiichi-Sankyo-Phase-3-trials?utm_campaign=GL_JP_update&utm_medium=email&utm_source=NA_newsletter&utm_content=article_link&del_type=4&pub_date=20210713090000&seq_num=2&si=44594 Japan nears homegrown vaccine with Daiichi Sankyo Phase 3 trials Shionogi eyes enough COVID shots for 60m people a year KENYA AKAMA, Nikkei staff writerJuly 13, 2021 04:44 JST TOKYO -- Daiichi Sankyo plans to begin Phase 3 clinical trials for its coronavirus vaccine this autumn with the aim of bringing it to the public in 2022, finally pushing forward Japan's effort to locally develop COVID-19 shots. A few other Japanese drugmakers are also making progress in vaccine development, including Shionogi, which says it would be able to supply enough doses for 60 million people a year if a reduced dose proves to be as effective as the previously proposed amount. Since March, Daiichi Sankyo has been conducting a combined Phase 1 and 2 trial of its vaccine based on the same type of messenger RNA technology with a group of about 150 adults. A Phase 3 clinical trial will involve thousands of volunteers. Typically in a Phase 3 study, half of participants receive the actual treatment and the rest get a placebo. But with the Pfizer and Moderna vaccines already available in Japan, a trial administering fake shots would raise ethical issues and turn volunteers away. So Daiichi Sankyo's next trial is expected to test non-inferiority, meaning that the goal is to show the company's new treatment matches or outperforms those made by Pfizer and Moderna -- also mRNA shots -- in terms of efficacy. Details of the study are being ironed out with the health ministry. Domestic subsidiary Daiichi Sankyo Biotech plans to establish a structure for producing the vaccine by March 2022. Capacity will be determined based partly on the results of the trials. Shionogi has been carrying out its trial since December. If its vaccine receives special conditional approval, the treatment may be rolled out before year-end, the company reckons. A factory in Gifu Prefecture is being updated to accommodate anticipated production. At the next stage of studies, Shionogi will test whether a smaller-than-planned dose of its vaccine has the same efficacy. If such dose stretching is proved effective, the vaccine's capacity will jump to as many as 60 million people a year, or twice the number anticipated initially. Shionogi is also readying large-scale Phase 3 trials in other markets such as Southeast Asia. Japan has no homegrown COVID-19 vaccine approved, and the country depends on overseas versions. Besides Daiichi Sankyo and Shionogi, two more domestic companies are conducting trials: KM Biologics, a group company of food giant Meiji Holdings; and biotech venture AnGes. KM Biologics intends to file for approval around summer 2022, with plans for a structure by that spring to churn out 35 million doses in six months.
-
San Francisco to 37,000 city employees: Get vaccinated for COVID or get out SAN FRANCISCO – In an announcement Wednesday evening, the city of San Francisco told its 37,000 employees they must either be vaccinated against COVID-19 within 10 weeks of the Food and Drug Administration giving final approval to a coronavirus vaccine, or lose their jobs. This would make San Francisco the first large U.S. city to require vaccination of all city employees. "It's quite straightforward – it's my job to protect the safety of our employees; I am exercising my duty under the San Francisco charter to do just that," said Carol Isen, the human resources director for the city and county of San Francisco. The vaccination policy released by the city on Wednesday said "failure to comply with this policy may result in discipline up to and including termination of employment." Currently, all COVID-19 vaccines being used in the United States were approved by FDA under what's known as an emergency use authorization, an expedited process. The emergency use is an authority that Congress gave to the FDA after the 9/11 terrorist attacks to allow countermeasures, treatments or vaccines to be available earlier than would be the case in a normal approval process. The full drug approval process takes longer. Some have used the fact that the Pfize, Moderna and Johnson & Johnson vaccines have all been issued under emergency use as a reason to doubt their safety. Pfizer and its German collaborator BioNTech submitted an application to the FDA for full approval of their COVID-19 vaccine on May 7. Moderna did so on June 1. The San Francisco policy requires all staff to report their vaccination status to the city no later than July 29 as a condition of employment. To do so, they must upload a copy of their COVID-19 vaccination card or documentation of vaccination from their health care provider.
- 15 replies
-
- 3
-

-
.png)
-
- vaccine
- vaccinated
-
(and 3 more)
Tagged with:
-
https://asia.nikkei.com/Spotlight/Coronavirus/COVID-vaccines/Hundreds-of-Sinovac-injected-Indonesian-doctors-contract-COVID?utm_campaign=GL_coronavirus_latest&utm_medium=email&utm_source=NA_newsletter&utm_content=article_link&del_type=10&pub_date=20210618123000&seq_num=15&si=44594 Hundreds of Sinovac-injected Indonesian doctors contract COVID Dozens hospitalized as concerns rise over efficacy against more virulent strains A medical worker prepares a dose of the Sinovac vaccine before giving it to a doctor at a facility in Jakarta, Indonesia, on January 19, 2021. © Reuters June 17, 2021 17:36 JST JAKARTA (Reuters) -- More than 350 Indonesian doctors have contracted COVID-19 despite being vaccinated with Sinovac, and dozens have been hospitalized, officials said, as concerns rise about the efficacy of some vaccines against more virulent virus strains. Most of the doctors were asymptomatic and self-isolating at home, said Badai Ismoyo, head of the Kudus district health office in Central Java, but dozens were in hospital with high fevers and declining oxygen saturation levels. Kudus is battling an outbreak believed to be driven by the more transmissible Delta variant which has pushed bed occupancy rates above 90% in the district. Designated as a priority group, Indonesian health care workers were among the first to be vaccinated when the inoculation drive started in January. Almost all have received the COVID-19 vaccine developed by Chinese biopharmaceutical company Sinovac, according to the Indonesian Medical Association (IDI). While the number of Indonesian health care workers dying from COVID-19 has decreased significantly -- dropping from 158 deaths this January to 13 this May, according to data initiative group LaporCOVID-19 -- public health experts say the Java hospitalizations are cause for concern. "The data shows they have the Delta variant [in Kudus] so it is no surprise that the breakthrough infection is higher than before because as we know the majority of health care workers in Indonesia got Sinovac, and we still don't know yet how effective it is in the real world against the Delta variant," said Dicky Budiman, an epidemiologist from Australia's Griffith University. A spokesperson from Sinovac and Indonesia's ministry of health were not immediately available for comment on the efficacy of Sinovac's CoronaVac against newer coronavirus variants. Grappling with one of the worst outbreaks in Asia, with more than 1.9 million cases and 53,000 deaths, there has been a heavy toll on Indonesia's doctors and nurses with 946 deaths. Many are now experiencing pandemic fatigue and taking an increasingly laissez-faire approach to health protocols after being vaccinated, said Lenny Ekawati, from LaporCOVID-19. "That phenomenon happens quite often these days, not only within the community but also health care workers," she said, "They think because they are vaccinated that they are safe." But as more cases of the highly transmissible Delta variant are identified in the world's fourth most populous nation, the data is starting to tell a different story. Across Indonesia, at least five doctors and one nurse have died from COVID-19 despite being vaccinated, according to the data initiative group, although one had only received their first shot. In Kudus, one senior doctor has died, said IDI, although it is understood he had a comorbidity. In the Indonesian capital Jakarta, radiologist Dr Prijo Sidipratomo told Reuters he knew of at least half a dozen doctors in the city who had been hospitalized with COVID-19 in the past month despite being vaccinated, with one currently being treated in ICU. "It is alarming for us because we cannot rely on vaccinations only," he said, urging people to strictly adhere to health protocols. Weeks after the Muslim Eid Al-Fitr holidays, Indonesia has experienced a surge in cases, with the positivity rate exceeding 23% on Wednesday and daily cases nearing 10,000, the highest since late February. In its latest situation report the World Health Organization called for Indonesia to implement a stricter lockdown with increased transmission due to variants of concern and a "drastic increase in bed occupancy rates" necessitating urgent action.
-
this is really good news after covid ... the mRNA technology will be accelerated to be used on many other applications/vaccines .... spore huat ah!
-
Personal and leisure travel will return from the second half of this year as borders reopen to tourists hungry to be free again and to reunite with families and friends, the director of the International Air Transport Association (Iata) said in an interview with The Straits Times. Mr Alexandre de Juniac said the recovery in business travel will be slower, and the actual volume of travel by the year end will still be low compared with the pre-Covid-19 period in 2019. "We will likely start seeing a change in the air travel landscape after May or June this year," he added. "We at Iata are already working with states to design and plan protocols and road maps for the reopening of borders." Singapore Airlines has been the first to officially announce that it will begin testing the Iata Travel Pass on flights from Singapore to London. Beginning Monday, passengers on that route using Apple iOS-enabled phones will be able to download the Travel Pass app and create a digital identification with their photo and passport information. https://www.straitstimes.com/business/economy/personal-travel-will-return-from-2nd-half-of-2021-iata-chief This will probably be the key driver for the vaccination take up rate. Humans will never stop wanting to travel. Being vaccinated does not guarantee you 100% immunity against the virus nor does it guarantee you will not cause the spread of the virus but I think the aviation industry, economies and people in general are getting desperate so we'll definitely be seeing more travel movements 2H onwards. Question is, will this cause another round of outbreaks? Is it really safe to travel so soon?
-
Covid has been with us for a while now and there are no signs that it will go away in the next few months. Social distancing, good hygiene, isolation and quarantine techniques have been instrumental in the initial battle and remain the primary weapons of choice in any outbreak. But now, we are seeing more countries resort to making vaccines in the hope that it can bring about stability and a return to normalcy. Having spent time in the trenches against this beast, I would like to share some info on the battle against it. R&D is now where the battle is being fought, and hopefully won.. However I must stress that a vaccine is no magic bullet, and all the above measures still apply and are very crucial regardless of the success of any vaccine. Second, the runway to creating a successful vaccine takes a lot of time - typically years and even with the accelerated development we are seeing now, it will probably be next year before we see any solid data and results. Third, not all vaccines are the same and the success may vary. With that, I'll just share some basic info on what a vaccine is, and then some info on what is the kind of R&D that goes into it and perhaps share info and get others to contribute on this matter, so we get real facts. I would like to emphasis once more that a vaccine is no magic bullet and the outlook for the rest of this year remains rather bleak... So What Is A Vaccine: I think the explanation on Wiki is the best answer that balances between too simple and too technical an answer: https://en.wikipedia.org/wiki/Vaccine